Medical
Delivering high-performance custom rubber components engineered to meet the exacting standards of patient care, regulatory compliance, and medical innovation.
The Rubber Group specializes in custom rubber molding solutions for the medical device industry. With decades of experience, we deliver precision-engineered rubber components that meet the stringent requirements of medical applications. Our commitment to quality, biocompatibility, and regulatory compliance makes us a trusted partner for medical device manufacturers, from startups developing innovative technologies to established OEMs supplying critical care equipment worldwide.
Key Applications
Fluid Delivery Systems
Precision-molded valves, seals, septums, and diaphragms for, infusion pumps, dialysis equipment, and fluid management systems that ensure accurate medication delivery and prevent contamination in critical care environments.
Surgical Instruments
Custom-engineered handles, grips, seals, and protective components for surgical instruments and power tools that provide ergonomic control, sterilization compatibility, and reliable performance in operating room environments.
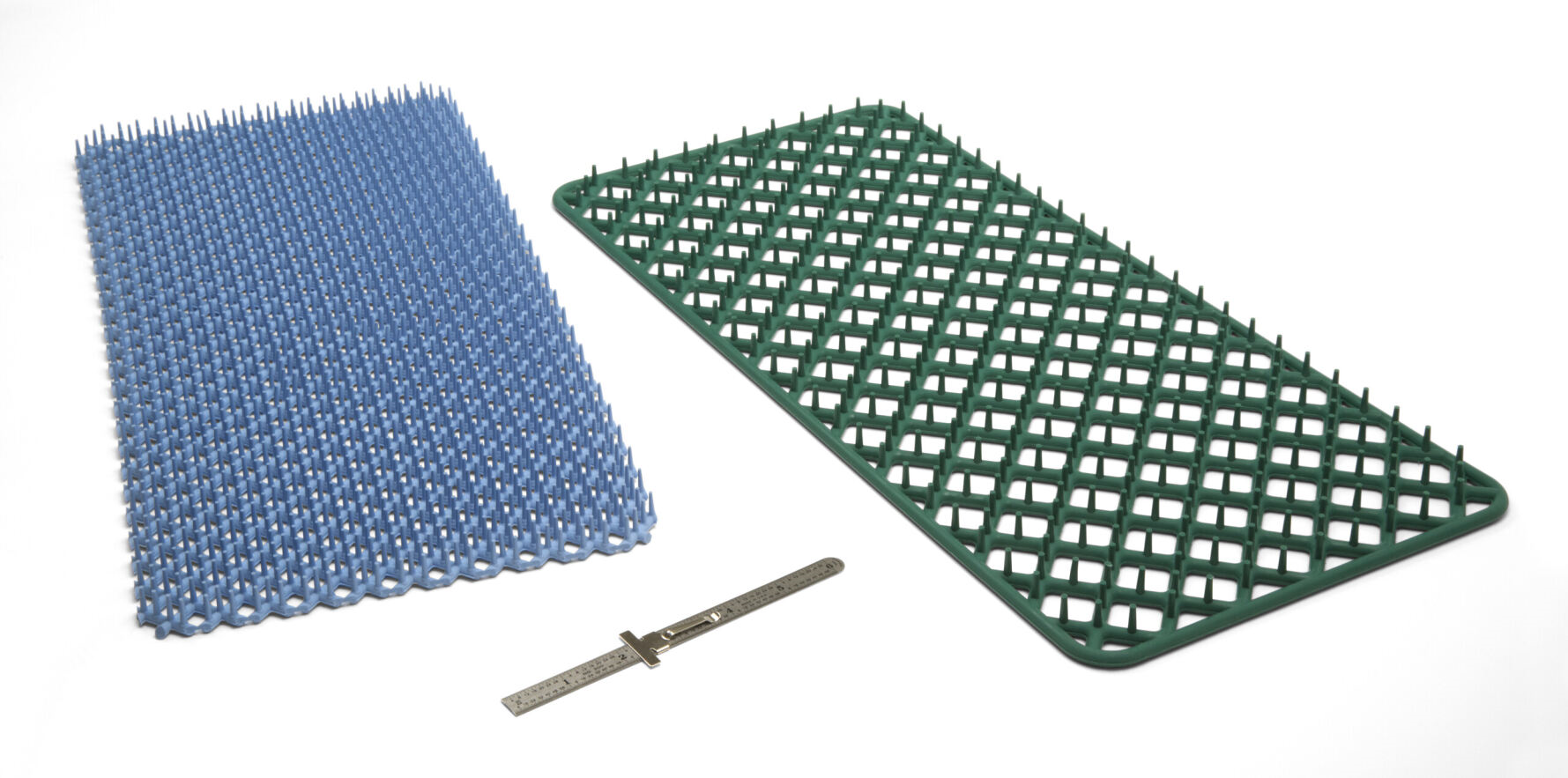
Sterilization Mats
Economical molded silicone pin mats used in sterilization processes. Various colors and hole patterns.

Diagnostic Equipment Components
High-precision seals, gaskets, and flexible elements for diagnostic equipment including blood analyzers, imaging systems, and point-of-care testing devices that maintain system integrity and ensure accurate results.
Respiratory & Anesthesia Products
Specialized gaskets, valves, and respiratory interface components for ventilators, anesthesia delivery systems, and respiratory therapy devices designed for patient comfort and gas pathway integrity.
Wearable Medical Devices
Biocompatible seals, adhesive components, and custom-molded interfaces for wearable monitoring systems, drug delivery devices, and patient monitoring equipment that ensure comfort during extended wear.
Relevant Registrations and Certifications
Full biocompatibility testing per ISO 10993 protocols
USP Class VI certification
Cytotoxicity, sensitization, and irritation testing
Extractables and leachables studies
Sterilization validation for EtO, gamma, steam, and other modalities
Custom color matching for medical device branding
FDA registered medical device manufacturing facility
Validated manufacturing processes with complete documentation
Electronic document control systems ensuring revision control
IQ/OQ/PQ validation processes for ultimate product qualification
Validated equipment and calibration systems
